保健・化学物質対策
The US EDSP21 Initiative
Leslie W. Touart, Ph.D.
Senior biologist
Office of Chemical Safety and Pollution Prevention
U.S. Environmental Protection Agency

The US Environmental Protection Agency (EPA) developed the Endocrine Disruptor Screening Program (EDSP) following passage of the Food Quality Protection Act (FQPA) of 1996 and subsequent amendments to the Federal Food, Drug, and Cosmetic Act (FFDCA) and Safe Drinking Water Act (SDWA). Specifically these statutes required EPA to “develop a screening program, using appropriate validated test systems and other scientifically relevant information, to determine whether certain substances may have an effect in humans that is similar to an effect produced by a naturally occurring estrogen, or other such endocrine effect as the Administrator may designate” (FQPA, 1996). In addition, “the Administrator may provide for testing under the screening program … any other substance that may be found in sources of drinking water if the Administrator determines that a substantial population may be exposed to such substance” (SDWA, 1996). Based on recommendations from the Endocrine Disruptor Screening, Testing, and Assessment Committee (EDSTAC; 1998); and pursuant to the Administrator's discretionary authority, EPA adopted a two-tiered screening and testing strategy known as the EDSP and expanded the program to include the androgen and thyroid hormonal pathways of the endocrine system and to address ecological effects.
EPA began issuing EDSP Tier 1 test orders for an initial list of primarily pesticide chemicals in 2009 and has begun reviewing the data from studies received. A second list of chemicals which will also include drinking water contaminants is being finalized for issuance of new test orders. EPA has now initiated a new initiative called EDSP21 to develop and refine high throughput screening (HTS) tools to support prioritization of chemicals to be tested for potential endocrine disrupting activity and to build confidence in HTS tools for eventual use beyond prioritization to use in actual EDSP Tier 1 Screening.
The objectives of the initiative are to maximize use of existing data, use targeted in vivo toxicity testing, use a variety of tools in a tiered testing and assessment framework, systematically add new tools and methodologies, and advance understanding of key events in endocrine toxicity pathways. The initiative is expected to address several challenges including an ability to assess a large number of chemicals which have many possible adverse outcomes, optimize the use of finite resources and time, and to meet public expectations for scientific soundness, transparency, and timeliness. The scientific tools used in assessing endocrine disruption are increasingly complex and changing, and new risk assessment and management challenges always arise.
The proposed EDSP21 Work Plan involves a multi-level and integrated approach that is illustrated in Figure 1 and further outlined in Figure 2. In the context of EDSP Tier 1 screening to determine whether or not a chemical has the potential to interact with E, A, or T, three main objectives are proposed:
(1)Prioritization
The near-term goal (<2 years) is to prioritize the universe of non-pesticide chemicals and information needs for pesticide active ingredients going through registration review using existing data, in silico models, and individual or suites of in vitro HTP assays to aid in determining the relative order in which chemicals should be screened.
(2)Screening (Tier 1)
The intermediate-term goal (2-5 years) is to replace current validated in vitro screening assays with validated in vitro HTP assays, use the results to inform and target current in vivo estrogen- or androgen-specific screening assays, and, where possible, reduce the use of animals for screening purposes.
(3)Replacement
The long-term goal (<5 years) is to consider full replacement of the in vivo screening assays with validated in vitro HTP assays and eliminate the use of animals for screening purposes.
It is anticipated that EDSP21 will improve the ability of EPA to meet the scientific challenges posed and administer its statutory obligations more easily and effectively.
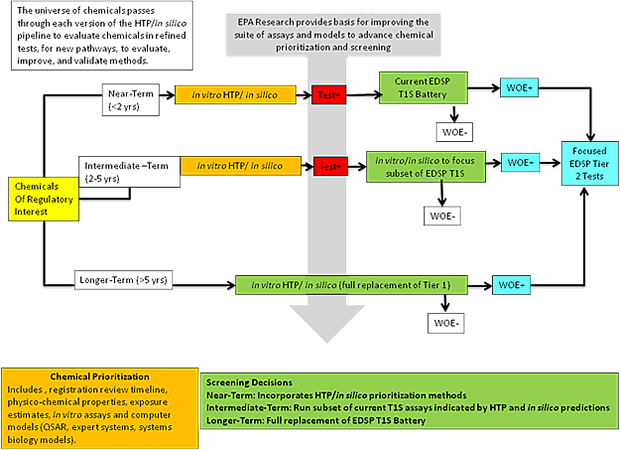
Figure 1: Illustration of the evolution of the EDSP Tier 1.
The non-pesticide chemical library could be analyzed using a battery of HTP assays. Computer models can be used as a prescreen method as appropriate. Chemicals that have been identified during this initial phase will be prioritized for the issuance of test orders under the current Tier 1 Screening (T1S) Battery. In the intermediate-term, chemicals would only be queued for T1S assays that are indicated based on the biological activity identified by HTP assays in vitro and in silico models. In addition, where appropriate, certain in vivo T1S assays would be replaced by one or a combination of validated in vitro/in silico models. The long term goal is to use information derived from in vitro, in silico and in vivo data to fully replace for the current EDSP T1S Battery such that animal-based testing is eliminated. WOE - weight of evidence evaluation for determining which chemicals need EDSP Tier 2 testing.
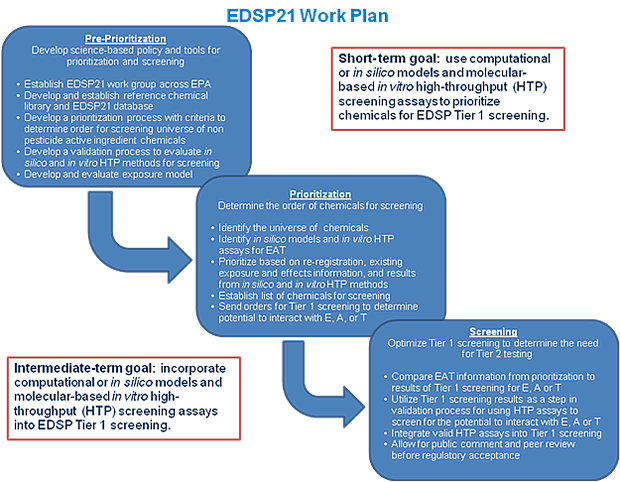
Figure 2: Work Plan activities that will enable reaching the Near-term and Intermediate-term goals.
Japanese
References
- EDSTAC (1998) Endocrine Disruptor Screening and Testing Advisory Committee, Final Report, Volume I-II.
- Food Quality Protection Act (FQPA) of 1996
- PresBudFY12EPA: Goal 4, page 61; Planning, Budget, and Results
- SDWA, 1996:
Bio for Leslie W. Touart, Ph.D.
Dr. Touart is a senior biologist with the U.S. Environmental Protection Agency in the Office of Chemical Safety and Pollution Prevention. He earned his Ph.D. in Environmental Biology and Public Policy from George Mason University. He leads the development of ecotoxicity tests for the Endocrine Disruptor Screening Program and participates in the development and harmonization of new ecotoxicity test guidelines with the Organization of Economic Cooperation and Development.