Nucleus Stability/Instability
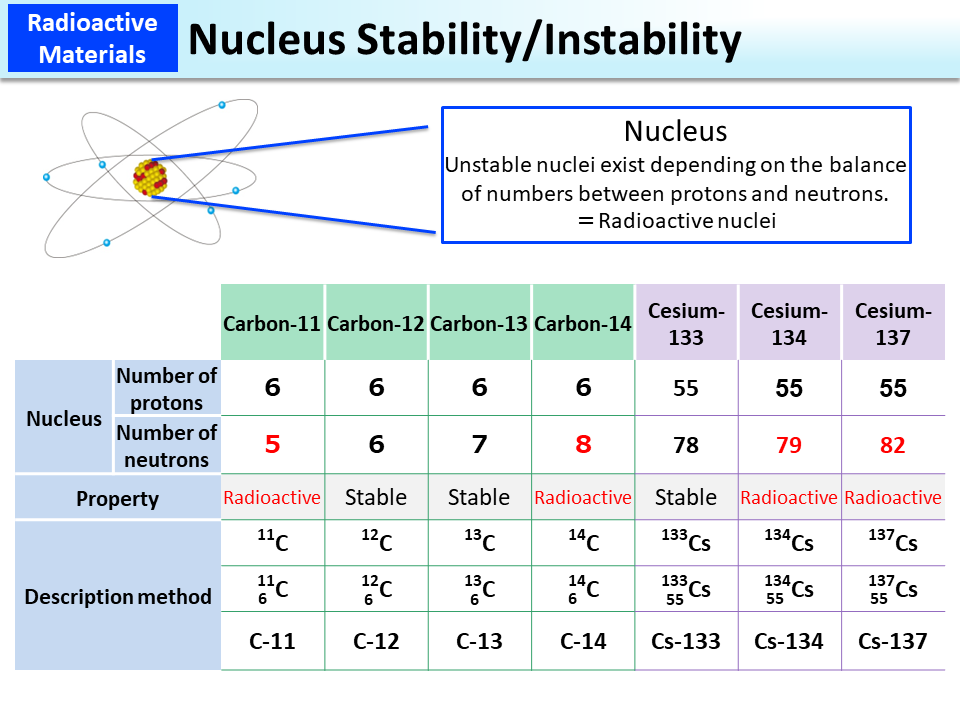
Nuclei having the same atomic number (the number of protons) but differing in the number of neutrons are called “isotopes” to each other. There are “radioisotopes” that emit radiation upon radioactive disintegration and “stable isotopes” that do not emit radiation and so do not change in atomic weight.
Radionuclides emit radiation such as α (alpha)-particles, β (beta)-particles, and γ (gamma)-rays to mitigate or terminate their unstable states. Radionuclides turn into different atoms after emission of α-particles or β-particles but such change does not occur after emission of γ-rays. The radiation type to be emitted is dictated for each radionuclide (p.8 of Vol. 1, “Naturally Occurring or Artificial,” and p.13 of Vol. 1, “Where does Radiation Come from?”).
Carbon is an element having six protons but there are also variants having five to eight neutrons. Cesium is an element having fifty-five protons, and its variants having fifty-seven to ninety-six neutrons have been found so far. Among them, only Cesium-133 having seventy-eight neutrons (55 protons plus 78 neutrons = 133) is stable, and all the rest are radioisotopes that emit radiation. In the event of a nuclear plant accident, Cesium-137 and Cesium 134 that is made through a process where fission products are hit by a neutron may be released into the environment. They emit β-particles and γ-rays.
(Related to p.30 of Vol. 1, “Products in Nuclear Reactors”)
- Included in this reference material on March 31, 2013
- Updated on March 31, 2019