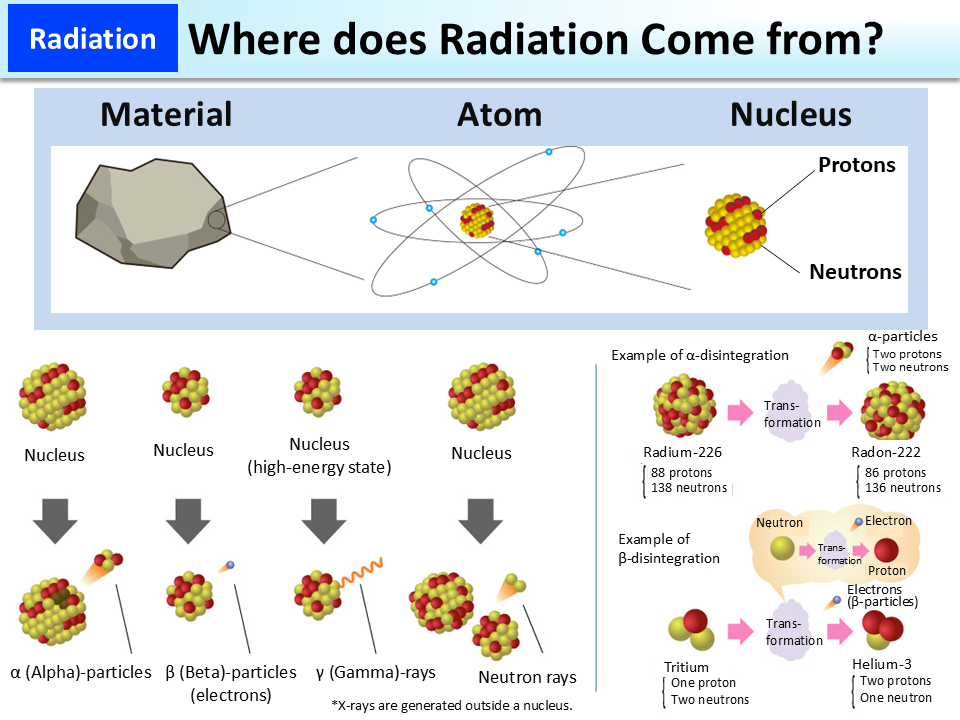
Where does Radiation Come from?
α (alpha)-particles, β (beta)-particles, γ (gamma)-rays, and X-rays were the names given to them because they were not elucidated at the time of their discoveries. Today, α-particles are found to be helium nuclei with two protons and two neutrons, flying out at high speed; β-particles are electrons that are ejected from a nucleus. A helium nucleus weighs about 7,300 times more than an electron. Normally, nuclei have high energy and are therefore still unstable immediately after emission of α-particles or β-particles, so they will further emit γ-rays in order to become stable. However, some do not emit γ-rays.
While α-particles, β-particles, and γ-rays are emitted from a nucleus, X-rays are electromagnetic waves that are generated outside a nucleus. Unlike X-rays, γ-rays are generated from a nucleus, but both are electromagnetic waves. A neutron is a particle that constitutes a nucleus. Neutrons that are ejected from a nucleus with kinetic energy, e.g. during the fission of the nucleus, are called neutron beams.
(Related to p.14 of Vol. 1, “Types of Radiation,” and p.15 of Vol. 1, “Types of Ionizing Radiation”)
- Included in this reference material on March 31, 2013
- Updated on March 31, 2019