Nuclei with Long Half-lives
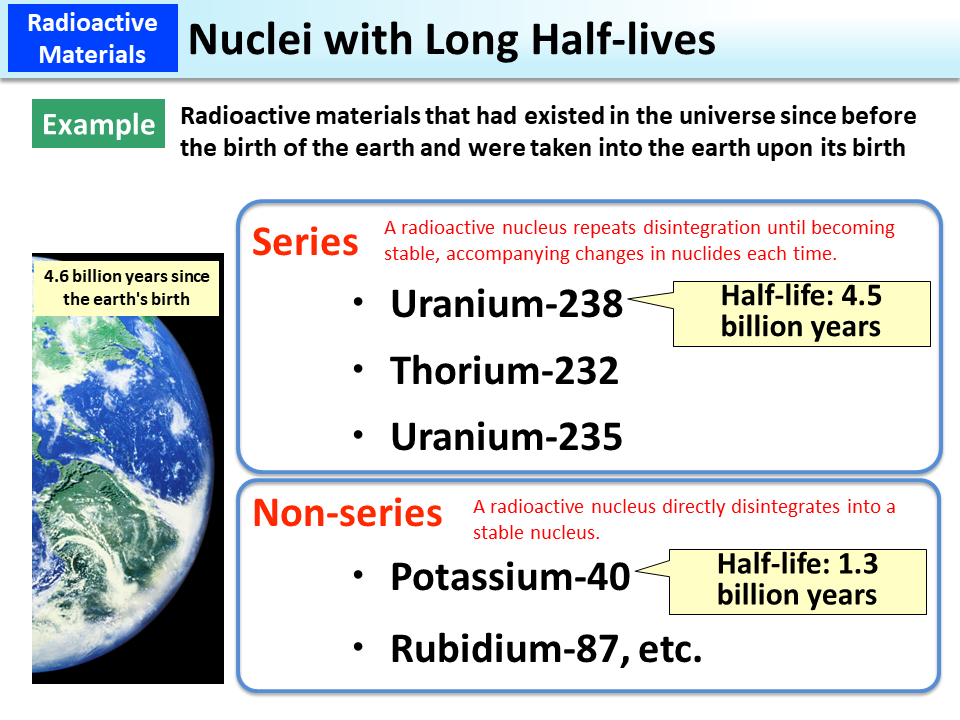
Some nuclei that emit radiation have very long half-lives. Uranium-238 has a half-life of 4.5 billion years. Since the earth is about 4.6 billion years old, the amount of Uranium-238 that had existed at the time of the earth's birth has now reduced to half.
Some radionuclides become stable after a single emission of radiation, while some transform into various radionuclides as they disintegrate many times, until becoming stable.
For example, Uranium-238 emits α (alpha)-particles and transforms into Thorium-234, which is also a radionuclide. Thorium-234 further emits β (beta)-particles and transforms into Protactinium-234, which is also a radionuclide. They constitute a series in which the original element transforms into different atoms more than 10 times before becoming stable Lead-206.
Potassium-40 also has a long half-life of 1.3 billion years. This is another naturally occurring radionuclide that was taken into the earth upon its birth. Potassium-40 transforms into stable Calcium-40 or Argon-40 through a single disintegration without constituting a series.
(Related to p.10 of Vol. 1, “Parent and Daughter Nuclides,” and p.11 of Vol. 1, “Half-lives and Radioactive Decay”)
- Included in this reference material on March 31, 2013
- Updated on March 31, 2019