Amount of Tritium Existing in the Environment
Tritium (3H) is a radioisotope of hydrogen (with a half-life of about 12.3 years) and emits weak radiation (β-particles) (p.79 of Vol. 1, “Characteristics of Tritium”).
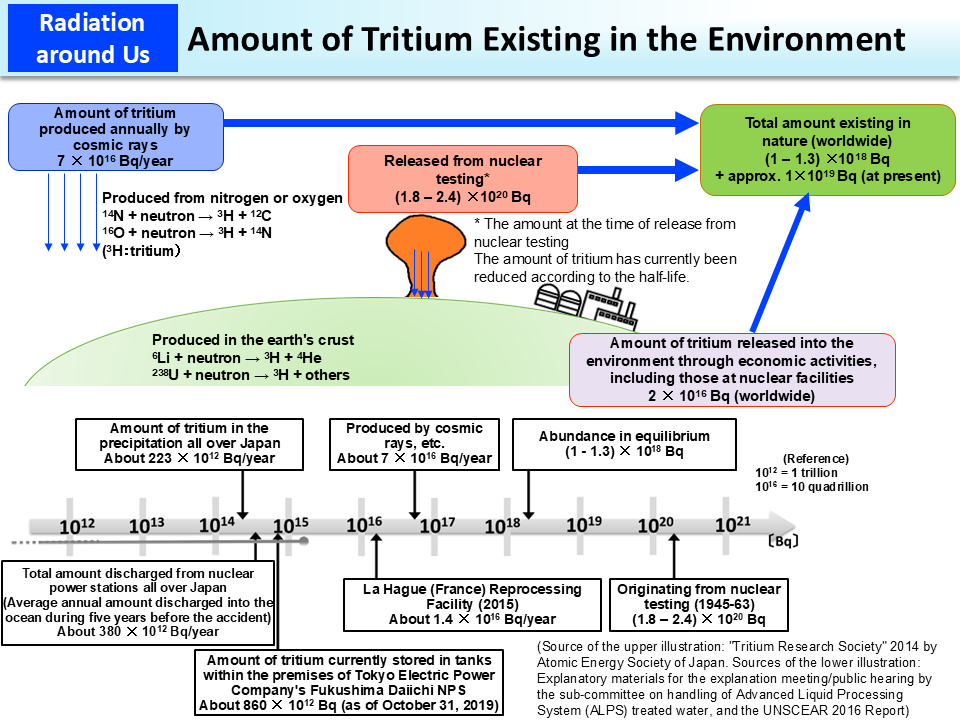
In nature, about seventy quadrillion (7 × 1016) Bq of tritium is produced annually by cosmic rays, etc. on earth. In the past nuclear testing (1945 to 1963), tritium of 1.8 to 2.4 × 1020 Bq was released. In addition, tritium is discharged daily from facilities such as nuclear power stations around the world and the annual amount of tritium released from nuclear power stations around the world is 2 × 1016 Bq. Before the Tokyo Electric Power Company (TEPCO)'s Fukushima Daiichi NPS Accident, the annual amount of tritium released from nuclear power stations all over Japan was 380 × 1012 Bq (which is the average annual amount discharged into the ocean during the five years before the accident). Abundance in equilibrium (abundance when generation and disintegration1 stay in equilibrium) in the environment is estimated to be 1 to 1.3 × 1018 Bq, and the current abundance of tritium derived from nuclear testing and released from nuclear facilities, etc. is estimated to be 1 × 1019 Bq. The released tritium exists mostly as hydrogen that makes up water molecules and it is also contained in water vapor in the atmosphere, rainwater, sea water and tap water. The annual amount of tritium contained in the precipitation in Japan is estimated to be about 223 × 1012 Bq.
- The phenomenon wherein a radionuclide emits radiation and transforms into a different nuclide (p.10 of Vol. 1, “Parent and Daughter Nuclides”). Tritium transforms into helium through disintegration.
- Included in this reference material on March 31, 2021
- Updated on March 31, 2024