Generation of Radon Gas from Solid Radium
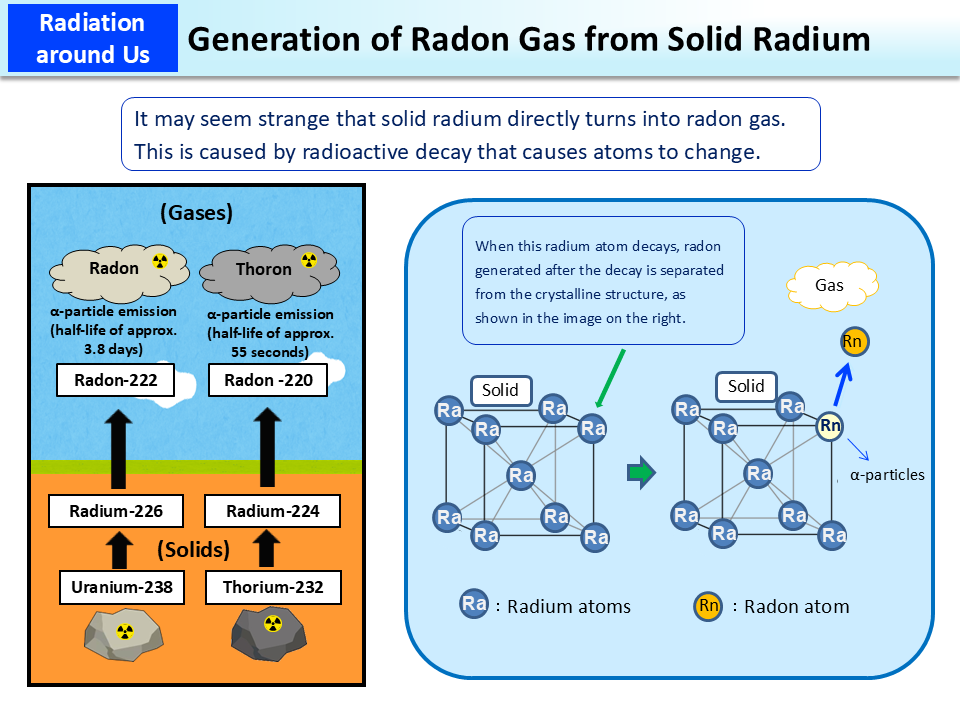
Radium, a radioactive material, is present in a crystal structure called body-centered cubic at room temperature and normal pressure, as shown in the right image.
When radium decays, it emits α (alpha)-particles and turns into radon.
Radon is a chemically stable element, like helium and neon. Being chemically stable or being an inert element means that it stably exists as radon without reacting with other elements to form compounds. Radon has a melting point of approx. -71°C and a boiling point of approx. -62°C and is therefore in a gas form under normal conditions. When radium atoms making up the crystal structure decay into radon atoms, they leave the crystal structure (because the force binding them as a crystal is lost) and come to exist in a gas form. Since radon is an inert gas, it emanates from the ground into the air without reacting with any underground substances.
- Included in this reference material on March 31, 2016