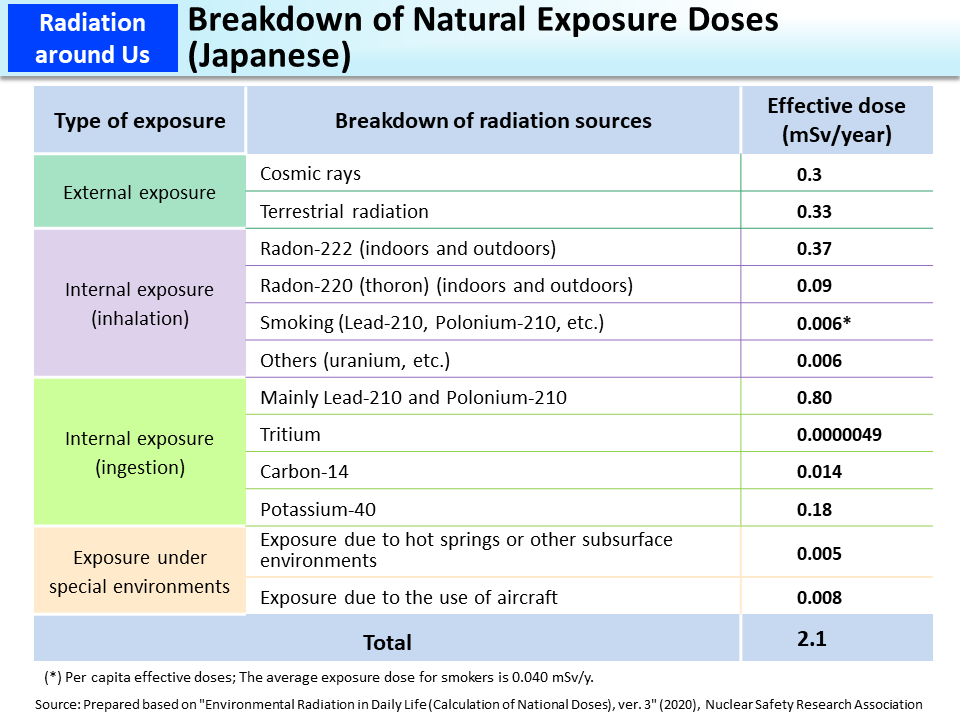
Breakdown of Natural Exposure Doses (Japanese)
This table shows that the intake of Lead-210 and Polonium-210 through ingestion accounts for a significant portion of Japanese people's internal exposures. Lead-210 and Polonium-210 are created when Radon-222 in the air goes through the following process. They are deposited on the ground or settled in rivers and oceans and are taken into the human body through foods.
Radon-222 (half-life of approx. 3.8 days) → Polonium-218 (half-life of approx. 3 minutes) → Lead-214 (half-life of approx. 27 minutes) → Bismuth-214 (half-life of approx. 20 minutes) → Polonium-214 (half-life of approx. 1.6 × 10-4 sec.) → Lead-210 (half-life of approx. 22 years) → Bismuth-210 (half-life of approx. 5 days) → Polonium-210 (half-life of approx. 138 days)
One reason why Japanese people's exposure doses from foods are higher compared to Western countries is that their diets contain lots of fish, which is abundant in Polonium-210. This accounts for Japanese people's large effective doses. Incidentally, analyses of Lead-210 and Polonium-210 in foods have rarely been conducted so often in foreign countries as in Japan and this is considered to be one of the factors of higher exposures to Lead-210 and Polonium-210 among the Japanese population when compared to the global average.
On the other hand, exposure to Radon is smaller among Japanese people, and this is considered to be due to the fact that the concentrations of Uranium-238, which generates Radon-222 through decay, are generally low in soil in Japan and that traditional Japanese houses are well ventilated and Radon-222 that seeps indoors from the ground is quickly diffused outside.
Internal exposure to Radon-222 and Radon-220 (thoron) through inhalation will be explained in “Internal Exposure to Radon and Thoron through Inhalation” on p.71 of Vol. 1.
Tritium has smaller effects on the human body compared with other nuclides, and the exposure doses due to natural tritium are relatively small (p.57 of Vol. 1, “Conversion Factors to Effective Doses”).
- Included in this reference material on March 31, 2013
- Updated on March 31, 2023