Ionization of Radiation - Property of Ionizing Radiation
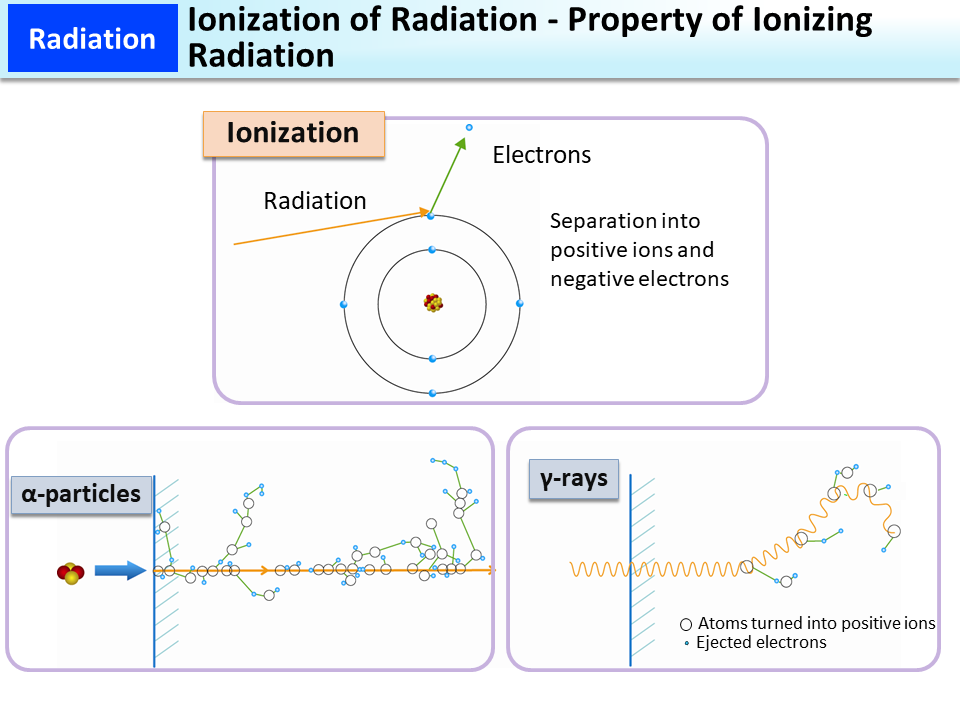
When radiation passes through a substance, its energy causes ejection of orbital electrons of the atoms that make up the substance, separating the atoms into positively charged atoms (or positive ion molecules) and free electrons. This is called ionization.
Ionizing radiation that causes ionization ionizes substances either directly or indirectly.
Charged particle beams, such as α-particles and β-particles, ionize substances directly. In particular, α-particles have high ionization density, causing ionization at a density hundreds of times as high as that of β-particles, etc.
γ-rays and X-rays ionize substances indirectly using secondary electrons generated through their interaction with the substances.
(Related to p.14 of Vol. 1, “Types of Radiation”)
- Included in this reference material on March 31, 2013
- Updated on March 31, 2015