Half-lives and Radioactive Decay
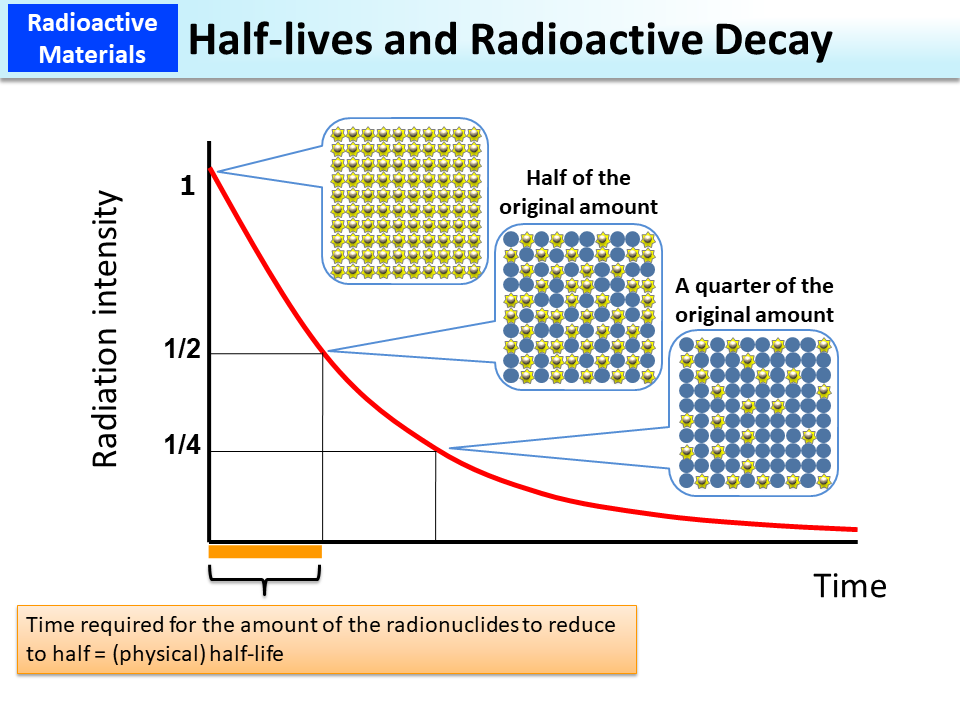
An atom that has become stable in terms of energy by emitting radiation will no longer emit radiation. The amount of a radionuclide decreases over time and radioactivity weakens. The time required for radioactivity to weaken and reduce to half is called a (physical) half-life.
Upon the elapse of a period of time equal to the half-life, the radioactivity will be halved, and when a period of time twice as long as the half-life lapses, the radiation will reduce to a quarter of the original state. A graph with the horizontal axis representing the elapsed time and the vertical axis representing the radiation intensity demonstrates exponential radioactivity decreases in a curve as shown in the slide.
(Physical) half-lives vary depending on the types of radionuclides. For instance, the half-life is approximately 8 days for Iodine-131, approximately 2 years for Cesium-134, and approximately 30 years for Cesium-137.
Radioactive materials taken into the body will be excreted after being taken into various organs and tissues. The time required for the amount of radioactive materials in the body to reduce to half through excretion is called biological half-life and varies depending on their chemical forms and/or particle sizes (p.27 of Vol. 1, “Internal Exposure and Radioactive Materials”).
- Included in this reference material on March 31, 2013
- Updated on March 31, 2019